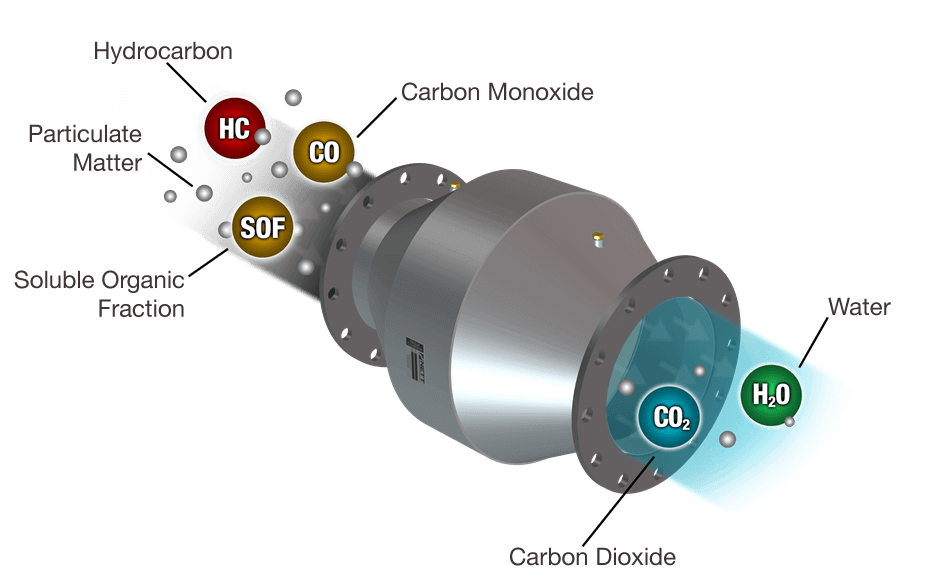
Catalytic oxidation of HC and CO
In the case of catalytic oxidation, partially burnt or unburnt exhaust gas components (e.g., carbon monoxide (CO) and hydrocarbons (HC)) are converted on the catalyst into carbon dioxide (CO2) and water (H2O).
Oxidation catalysts are mainly based on precious metal coatings. we tend to use platinum and palladium. The coatings are applied to honeycomb substrates made of ceramic materials or metal.
Greenhouse applications (COdiNOx) are extremely demanding when it comes to reducing hydrocarbons (ethylene C2H4). This is because ethylene is highly poisonous for plants and therefore has to be removed almost completely from the exhaust gas mixture. Greenhouse-based systems have to achieve values of less than 300 ppb throughout the entire life of the catalyst, which can be several tens of thousands of hours.
The methods used by is to reactivate used oxidation catalysts can deliver results in excess of 95% of those obtained from a new catalyst.
The DOC (diesel oxidation catalyst) is a special type of oxidation catalyst. It converts nitrogen monoxide (NO) into nitrogen dioxide (NO2) so that passive soot reduction can take place in the filter (CRT process).